Research Article
Effect of Different Doses of Rabbit Manure on the Abundance, Growth Rate and Production of Zooplankton in Plurispecific System for Fish Larvae Feeding 
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2017, Vol. 7, No. 17 doi: 10.5376/ija.2017.07.0017
Received: 20 Sep., 2017 Accepted: 12 Oct., 2017 Published: 27 Oct., 2017
Adande R., Liady M.N. D., Sossoukpe E., and Fiogbe E.D., 2017, Effect of different doses of rabbit manure on the abundance, growth rate and production of zooplankton in plurispecific system for fish larvae feeding, International Journal of Aquaculture, 7(17): 112-121 (doi: 10.5376/ija.2017.07.0017)
In order to determine the optimal dose of rabbit manure for plurispecific production of fresh water zooplankton in controlled area, the effects of six different doses of dry rabbit manure (T1 = 300 g/m3, T2 = 600 g/m3, T3 = 900 g/m3, T4 = 1200 g/m3, T5 = 1500 g/m3, T0 = 0g/m3) on physico-chemical and biological parameters of areas were monitored for 27 days in cultures realized in plastic buckets of 80 L capacity each. Manures are from rabbits fed with diet previously revealed favorable to their growth, and is made of 2% of dried cassava, 30% of maize bran, 10% of palmist cake, 10% of soya cake, 5% of cotton cake, 2% of shell, 10% of malt, 5% of beer yeast, 10% of Panicum maximum and 1% of salt. Six days after fertilization, buckets were seeded with zooplankton by 26 individuals per liter (ind/L). Results obtained show that the supply of rabbit manure has improved chemical properties and microalgae production (phytoplankton) with treatments T1, T2, T3, T4 and T5. Optimal conditions of zooplankton production through phytoplankton are obtained with doses in T2 and T4 which present the best specific growth rate. The daily production through the highest phytoplankton biomass (chlorophyll-a) has been obtained with dose in T5. Thus, doses 600 g/m3 and 1200 g/m3 of rabbit manure may be considered optimal for a plurispecific production of fresh water zooplankton. It was noted that Rotifers were predominant in abundance in almost the different culture areas.
1 Background
The natural feed appreciated by fish larvae is essentially made of zooplankton and artificial dry feed are not always appropriated to this premature stage (Arimoro, 2005). Consequently, zooplankton such as cladocerans, copepods and rotifers are used for larval rearing by many researchers in controlled area (Awaïss et al., 1992). Artemia (live food) is the most used for certain marine species whose hatching, development and growth were realized in pond (Agadjihouèdé et al., 2011). Nevertheless, it remains less effective in fresh water fish larvae. Artemia as larvae food is inaccessible to rural fish farmers (Fiogbe et al., 2003). Indeed, its use doesn’t always give good zootechnical performances to fresh water fishes. Zooplankton production in controlled area is carried out in many studies, by the way of phytoplankton production using different types of fertilizer (chemical, organic genuine vegetal or animal) (Adande et al., 2015). Chemical fertilizers (minerals) are the former used for phytoplankton production for the purpose of zooplankton production (Boyd et al., 2008) while organic fertilizers genuine vegetal or animal are the second used (Dalme et al., 2011). Between these two categories of fertilizer, the first type is dangerous to the environment and also expensive while the second type is less expensive and does not present any risk for the environment (Agadjihouèdé et al., 2011; Adande et al., 2017). These findings justify nowadays the easy use of organic fertilizers genuine animal such as cow and poultry manure (Agadjihouèdé et al., 2010), and pig dung (Ekelemu et al., 2011), in zooplankton production. The quantities of these organic fertilizers used influence too much the productivity of phytoplankton and indirectly of zooplankton and; according to the literature; there is an optimal dose for which a maximum production of zooplankton could be obtained. Indeed, different doses of manure have been tested in experiments on zooplankton production by many authors. Saint-Jean et al. (1994), for the production of Moina micrura used 0.05 g of poultry manure per liter of water; Agadjihouèdé et al. (2010) used in aquarium designed for zooplankton production 0.6 g of poultry manure per liter of water; by the same way, Akodogbo et al. (2014) used 0.3 g of pig dung per liter of water in plastic buckets during a plurispecific process of zooplankton production. Indeed, high production and survival rate of fish larvae are realizable from a low cost production of nutritive algae and zooplankton (Sipaúba-Tavares et al., 2007). That is the reason why, the aim of this study is to carry out the plurispecific production of fresh water zooplankton for larvae of Clarias gariepinus and Heterobranchus longifilis. The study looks to evaluate the progression of zooplankton density as a function of different doses of rabbit manure.
2 Materials and Methods
2.1 Experimental site
The experiment was conducted on the Diversification of Fish Farming Station of the Laboratory of Research on Wetlands that is located in the Department of Zoology of the Faculty of Science and Techniques, University of Abomey-Calavi (Benin). It is located between 6° 24' 53.3'' N; 2° 20' 18.5'' E and 6° 24' 53.4'' N; 18.5'' E and 9 m.a.s.l.
2.2 Experimental design
The experimental design is made of 18 plastic buckets of 80 L capacity each, exposed at free air on the previously described site. For this purpose, seventy-two hours before experiment starting up, buckets were cleaned and disinfected. It is made of six (06) treatments including one control such as: T0, T1, T2, T3, T4, T5 corresponding respectively to 0, 300, 600, 900, 1200, 1500 g of dry rabbit manure (RM) in one (01) m3 of water replicated thrice. Buckets were filled with 40 liters of demineralized water and immediately fertilized. Manures are from rabbits fed with a low price diet but provided good performances on rabbits rearing (Adande et al., 2017; in press) and is made of 2% of dried cassava, 30% of maize bran, 10% of palmist cake, 10% of soya cake, 5% of cotton cake, 2% of shell, 10% of malt, 5% of beer yeast, 10% of Panicum maximum and 1% of salt. Manures contained 15.02%, 1.26% and 0.84% of N, P and K respectively.
2.3 Phytoplankton seeding process
After fertilizing, production areas were left for three days in order to enable nutrients releasing by washing so that they will be available to phytoplankton (microalgae). After this period, areas were seeded with phytoplankton according to Agadjihouèdé et al. (2010) and Akodogbo et al. (2015) by transferring ten (10) liters of polyculture (Clarias and Tilapia) pond water previously filtered with plankton net of 25 µm mesh to eliminate zooplankton.
2.4 Zooplankton seeding process
Zooplankton was seeded three (03) days after microalgae seeding, otherwise six days after fertilizing. Indeed, according to Guiral et al. (1994), this period is sufficient for microalgae development. To harvest zooplankton, three hundreds (300) liters of the previous pond were filtered with a net of 25 µm, and then concentrated in 500 mL of water. Each bucket was seeded with 25 mL of this filtrate. A sub-sampling of 50 mL was taken and formolled in order to perform individuals counting and zooplankton diversity study. The initial seeding density was 26 ± 0.23 ind/L otherwise (5 ± 0.13 ind/L nauplii of copepods (Thermocyclops sp.), 4 ± 0.23 ind/L adults of copepods (Thermocyclops sp.), 2 ± 0.14 ind/L of cladocerans (Moina sp. and Daphnia sp.) and 15 ± 0.41 ind/L of rotifers (Brachionus sp. and Asplanchna sp.). These three great groups of zooplankton are generally phytophage. According to Sprules (1980) and Pourriot et al. (1982), a great number of copepods become carnivorous at a given stage of their development. After its seeding on day 0 (D0), zooplankton growth in different treatments was monitored every three days, at 8 a.m., for 24 days. During this period, a sampling was carried out every three days in order to follow biomass progression. Each sampling of zooplankton was preceded by homogenization of the culture, and then a taking of five (05) liters of water that are filtered with plankton net (25 µm mesh). The harvest was preserved according to Ara (2001) with 4-5% of formol in bottles of 100 mL until its analysis.
2.5 Image capture and zooplankton counting
Zooplankton counting was carried out according to Liady et al. (2015), by image treatment using Image Pro-Plus® software (Figure 1). Pictures were first captured from a microscope Optim Jeulin (X10) equipped by a photograph device (Figure 2). The technique of image treatment offering possibility to enlarge images during counting process enabled to distinguish easily rotifers from 120 µm. Different individuals of zooplankton were counted following species representativeness (at least 100 individuals were counted) in 1-2 mL of sample that were successively observed in a plastic saltcellar at densities that do not provoke overlapping among individuals.
|
Figure 1 Zooplankton counting process |
|
Figure 2 Technique of image capture |
2.6 Physico-chemical and trophic parameters
During experiment, temperature, pH, conductivity and dissolved oxygen have been measured in situ every sampling day at noon with a multi-parameter borer CALYPSO (Version Soft/2015, SN-ODEOA 2138) à ± 0.1°C and by mg/L). At each sampling site, 500 mL of the culture was filtered per bucket on the one hand for dosage of chlorophyll-a and for dosage of nutritive salts (ammonium, nitrate, nitrite and orthophosphate) on the other hand. Dosage of chlorophyll-a was carried out according to SCOR-UNESCO (1966) while dosage of nutritive salts was realized according to Rodier et al. (2016), with a spectrophotometer of molecular absorption (HACH DR/2800).
2.7 Comparison of different treatments performances
Performances of the six treatments were compared following two methods:
The first consisted to the comparison of different densities obtained in treatments; the second consisted to compare specific growth rates of organisms during their exponential growth stage according to Liady et al. (2015). In this second method, growth curves of zooplankton in non- renewed area presenting identical stages to those observed in microorganism’s cultures in non-renewed area, identical mathematical laws were considered. Thus, in each treatment, the exponential growth stage of density was considered and described by the formula (Waldbauer, 1968). From this formula, specific growth rate (µ) was determined by monitoring the progression of the logarithm of the relative density as a function of the time; it corresponds to the slope of the straight line obtained.
2.8 Statistical analysis
From data collected, density (D) and daily production (P) were obtained respectively by using these equations:
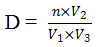
.png)
Where n = number of individuals counted; V1 = volume of aliquot; V2 = volume of sample concentrated; V3 = volume of water filtered; D0 = initial population density; Dt = population density at time t (in hour)
Collected data were analyzed using STATISTICA software (Statsoft inc., Tulsa, OK, USA). All significant levels were fixed at P<0.05. The influence of treatments was studied using a one-way ANOVA; in case of need, significance of differences among means was tested using the test LSD of Fisher.
3 Results
3.1 Water physico-chemical parameters
Different physico-chemical parameters of treatments varied strongly (Table 1 and Figure 3) during experiment period except temperature and pH which remained relatively constant. By the same way, conductivity is high in fertilized media with a significant difference among treatments (F(5, 12) = 33.92; p<0.00). Dissolved oxygen (DO) is most important in treatments T2 and T5 followed by T4 and T3 compared to T0 with a significant difference (F(5, 12) = 69.42; p<0.00) (Table 1). This oxygen concentration shows the photosynthetic activity of algae and consequently their presence in culture media. Moreover, salinity and TDS followed the same trend with a significant difference between T0 and the other treatments respectively (F(5, 12) =11.60; p<0.00 and F(5, 12) = 7.79; p<0.00). Besides, the high value of transparency of Secchi’s disk in treatment T0 contrary to others explained clearly its low proportion in suspended matter and algae. Concentrations of N-NH3 obtained were important in all treatments except T0 and varied between 0.06 ± 0.12 mg/L (T0) and 2.82 ± 0.75 mg/L (T5) (Figure 3). Concerning mean values of nitrite and nitrate, they varied respectively between 0.07 ± 0.11 mg/L and 0.08 ± 0.1 mg/L for T0; 0.75 ± 0.35 mg/L and 0.81 ± 0.14 mg/L for T5 (Figure 3). By the same way, orthophosphate concentration is high in all treatments except in T0 and followed the same trend with a mean value in range of 0.5 ± 0.13 mg/L (T0) and 3.15 ± 0.16 mg/L (T5) (Figure 3). These different concentrations of in the different treatments were highly significant (F(15, 36) = 842.99; p<0.00). It results from this analysis that the organic fertilizer (rabbit manure) used had strongly affected the water quality of different culture areas in plurispecific production of fresh water zooplankton. Thus, it could be used as a fertilizer for primary production.
|
Table 1 Physico-chemical parameters of different treatments Note: Cond = conductivity, DO = dissolved oxygen, Sal = salinity, Transp = transparency, TDS = total dissolved solids. Mean values affected by the same letter on the same line are not significantly different at the threshold of 5% according to LSD of Fisher |
|
Figure 3 Nutrients proportion in different culture middle |
3.2 Trophic parameter
Figure 4 expresses different concentrations of chlorophyll-a obtained during experiment in each treatment. There was a high significant difference among treatments (F(40, 96) = 1003.5; p<0.00). Also, we observed a strong linear correlation between the increase of chlorophyll-a rate and different doses of rabbit manure (R = 0.88; R² = 0.78; R² Adjusted = 0.76; F(1, 16) = 55.608; p<0.00) tested. These different phytoplankton biomasses could serve for fresh water zooplankton development and indirectly for fish production.
|
Figure 4 Chlorophyll-a rate of different treatments |
3.3 Density progression and phytoplankton production
Treatments T2 and T4 showed the best densities of zooplankton. Indeed, the density passed from 15 ind/L to 12,207 ind/L for rotifers (Brachionus sp. and Asplanchna sp.) and 3467 ind/L for eggie rotifers. There was a significant difference among treatments (F(7, 84) = 41,685; p<0.00). Similarly, the density increased from 2 ind/L to 840 ind/L of cladocerans (Moina sp. and Daphnia sp.) with a significant difference (F(7, 84) = 738.18; p<0.00), but there was no difference between T0 and T1 (p>0.00). Nauplii of copepods (Thermocyclops sp.) increased from 5 ind/L to 140 ind/L with a significant difference (F(7, 84) = 329.10; p<0.00) between T2 and T3 on the one hand, and T1 and T0 and the other treatments on the other hand. However, there was no significant difference among T2, T4 and T5 (p>0.00). In addition, density of adult copepods (Thermocyclops sp.) increased from 4 ind/L to 260 ind/L with a significant difference (F(5, 12) = 47.15; p<0.00) and followed the same progression trend as nauplii. Besides, treatment T0 (non-fertilized) presented a low density regarding different groups of zooplankton, what could be explained by the impoverishment of the area in nutritive elements. Rotifers, adult copepods and cladocerans reached their optimum at the 9th day while nauplii of copepods and eggie rotifers reached their optimum at the 6th day (Figure 5). The decrease of the density began on the 12th day in all treatments and the 9th day for nauplii and eggie rotifers (Figure 5B; Figure 5D). Figure 5F presents the total density of zooplankton in different treatments.
|
Figure 5 Variation of zooplankton densities in different treatments |
3.4 Effect of treatment on the production and specific growth rates of zooplankton
Daily production of different group of zooplankton per treatment is reported in Table 2. Thus, the daily production was higher in all fertilized treatments than in the control (T0) (p<0.05) (Table 2). The highest production was obtained in treatment T2 followed by T4 (Table 2). This high production of different groups of zooplankton in T2 revealed that this area offered the most suitable conditions to the development of these organisms. Specific growth rates of different groups of zooplankton determined on the exponential growth stage are mentioned in Table 2. Their comparison showed a high significant difference among treatments regarding rotifers (F(5, 12) = 6,568.6; p=0.00), cladocerans (F(5, 12) = 25,705; p=0.00) and copepods (F(5, 12) = 3,586.5; p=0.00). The best specific growth rates (µ) were obtained in treatment T2 followed by T4 (Table 2). From the multiple analysis of treatments effect (test LSD of Fischer), it results that treatment T2 followed by T4 presented the best specific growth rates.
|
Table 2 Production and specific growth rates of different groups of zooplankton Note: µ = specific growth rate, R2 = coefficient of correlation, P = daily production. Means followed by the same letter are not significantly different (P>0.05) according to the LSD test of Fisher |
The supply of rabbit manure as organic fertilizer would be the source of this performance of zooplankton.
4 Discussion
The mean temperature in buckets surrounded 34.51 ± 1.78°C during the full period of experiment with a pH value in the range of 6.87 ± 0.94 and 7.33 ± 0.94. The limit of tolerance of fresh water zooplankton (rotifer for instance: Brachionus calyciflorus) is in the range of 15 and 31°C. The optimal value of pH is situated between 6 to 8 (Ludwig, 1993). Temperatures recorded during experiment were close to those found by Park et al. (2001) and Kabir et al. (2010) that fluctuate between 28 and 32°C during fish larvae feeding with great densities of rotifers. The conductivity was function of the area richness in minerals (NH4+, PO4-, NO3-, NO2-). It was higher than those obtained by Akodogbo et al. (2015) with pig dung and close to what was reported by Bokossa et al. (2014) with pig manure. This difference may be explained by the greatest richness of rabbit manure (compared to pig dung) in mineral salts necessary to primary production that is the base of organisms’ development. These results are comparable to those obtained by Agadjihouèdé et al. (2011) with poultry manure. Indeed, the use of rabbit manure had significantly increased nutrients concentration in the water and accordingly enabled a good primary production for zooplankton development. These results are comparable to those obtained by Dalme et al. (2011) during zooplankton production process from the use of animal wastes. The effects of rabbit manure are revealed by the concentration of chlorophyll-a in treatments T1, T2, T3, T4 and T5. Concentration of chlorophyll-a obtained in these different treatments were higher than those reported by Canovas et al. (1991) that is 2 mg/L as minimal value for a good phytoplankton production. Thus, treatments T1, T2, T3, T4 and T5 are favorable to zooplankton development. The most important zooplankton concentration was obtained in treatment RM1500 and could be explained by its strong load in organic matter due to the strongest concentration of orthophosphate and ammonium. Indeed, phosphorus in the shape of orthophosphate (assimilable) is the prime element required for vegetal (algae) development (Reynold, 1980; Schlumberger et al., 2002). In addition, the low transparency value obtained in treatments T2, T3, T4 and T5 indicate the strong load in algae in treatments these areas particularly in T3 and T5. This great load could be the source of low density recorded in these treatments. However, oxygen levels recorded in different doses were slightly higher than those recorded by Agadjihouèdé et al. (2011), in the range 5.5±0.9 and 6.10±1.21. Such difference could be due to different in time of parameters measurement. Highest chlorophyll-a rates were obtained in doses of T5 though, the density of zooplankton was low. Reversely, the treatment T2 provides excellent nutritive conditions to zooplankton.
4.1 Production and specific growth rates of zooplankton
Preferable densities of zooplankton were given by doses in T2 and T4 (600 g/m3 or 0.6 g/L and 1200 g/m3 or 1.2 g/L). Several studies were based on the relationship between algae biomasses and zooplankton production (Sendacz et al., 2006; Ekelumu et al., 2011). This relationship could be characterized by optimal concentrations lower than which zooplankton growth is exponential and upper than which this growth is inhibited (Ovie et al., 2002; Liady et al., 2015). This matter could explain low productions of zooplankton observed in T5 and T3 compared to those observed in T2 and T4. The inhibition of zooplankton growth by high biomasses of algae might be due to their respiration (especially at night) that provokes zooplankton asphyxia. Indeed, high densities of algae tend to impact negatively the availability of oxygen for zooplankton that is competed by bacteria that also need it to deteriorate (mineralize) rabbit manure in order to make nutritive elements available to algae. In addition, the development of an important algae biomass is not sufficient for good fish farming; the nature of algae species and their height is to be considered (Lazzaro et al., 1995; Barbe et al., 2010). In the same way, treatments T2 and T4 provided the best specific growth rates with a best daily production among the different zooplankton species in culture areas with an abundance of rotifers. Indeed, this abundance of rotifers is a trump for larval rearing by considering their small height in relation to the diameter of larvae muzzle. Specific growth rate recorded were upper than those obtained by Agadjihouèdé et al. (2014) that are 0.74, 0.85 and 0.21 respectively for Brachiomus calyciflorus, Moina micrura and Thermocyclosps sp. This difference could be explained by the nature of fertilizer that is function of the quality of the animal diet. It results from this analysis that treatment T2 (600 g/m3) would be the favorable dose for an optimal plurispecific production of fresh water zooplankton.
5 Conclusion
This experiment showed that the doses of 600 g/m3, 1200 g/m3 and 1500 g/m3 in rabbit manure provided very good zooplankton productions. Nevertheless, the doses of 1200 g/m3 and 1500 g/m3 could provoke eutrophication of the culture area. For an optimal production of zooplankton, the dose of 600 g/m3 in rabbit manure offering the best conditions for zooplankton production could constitute the optimal dose of dry rabbit manure to be proposed in controlled area. Being aware that of the major challenges in fish farming is the feeding at different growth stages, it could be suggested the possibility to adopt different doses of rabbit manure as a function of the individual height of zooplankton to be produced in majority. Unfortunately, with almost the different doses applied, it was noted that rotifers were predominant in abundance.
Acknowledgements
We express our gratitude to the Ministry for the Higher Education and Scientific Research of Benin, which set up the project “support at PhD student” in which this study of research was made. We thank, Azon M.T. Césaire for her contribution to this work, particularly in data collection. The authors thank also the reviewers for their contribution in improve the scientist quality of this manuscript.
Adande R., Bokossa H.K.J., Liady M.N.D., and Fiogbe E.D., 2017, Valorization of various sources of rabbit manure in agro-piscicultural system in Benin (West Africa): dynamics and effect of mineralization upon quality of fresh water, International Journal of Recycling of Organic Waste in Agriculture, 6(3): 233-243
https://doi.org/10.1007/s40093-017-0170-x
Adande R., and Fiogbe E.D., 2015, Utilisation des fertilisants organiques d'origine animale et végétale pour le développement de la pisciculture dans les étangs: Synthèse bibliographique, International Journal of Multidisciplinary Research and Development, 2 (12): 281-287
http://www.allsubjectjournal.com/download/1672/2-12-92.pdf
Agadjihouèdé H., Bonou A.C., Montchowui E., and Laleye P., 2011, Recherche de la dose optimale de fiente de volaille pour la production spécifique de zooplancton à des fins piscicoles, Cahiers Agricultures, 20(4): 247-260
https://doi.org/10.1684/agr.2011.0495
Agadjihouèdé H., Bonou C.A., Chikou A., and Lalèyè P., 2010, Production comparée de zooplancton en bassins fertilisés avec la fiente de volaille et la bouse de vache, International Journal of Biological and Chemical Sciences, 4(2): 432-442
https://doi.org/10.4314/ijbcs.v4i2.58143
Agadjihouede H., Montchowui E., Montcho S.A., Bonou C.A., and Laleye P.A., 2014, Growth and development of three species of the zooplankton (Brachionus Calyciflorus, Moina micrura and Thermocyclops sp.) breeding on poultry dropping in mixed condition in tanks, International Journal of Fisheries and Aquatic Studies, 2(1): 189-196
http://www.fisheriesjournal.com/archives/2014/vol2issue1/PartD/47.pdf
Akodogbo H.H., Bonou C.A., and Fiogbe E.D., 2014, Effect of pig dung fertilizer on zooplankton production, Journal of Applied Biosciences, 84(1): 7665-7673
https://doi.org/10.4314/jab.v84i1.7
Akodogbo H.H., Bonou C.A., Adandé R., Sossou D.S., and Fiogbé E.D., 2015, Optimization of zooplankton production from pig dung optimal dose: renewed medium, Agricultural advances, 4(2): 15-21
http://www.jebas.org/00300120022015/AKODOGBO%20et%20al%20JEBAS.pdf
Ara K., 2001, Length-weight relationships and chemical content of the planktonic copepods in the Cananéia Lagoon estuarine system, Sao Paulo, Brazil, Plankton Biol. Ecol, 48(2): 121-127
http://plankton.jp/PBE/issue/vol48_2/vol48_2_121.pdf
Arimoro, F., 2007, First feeding in the African Catfish Clarias anguillaris fry in tanks with the freshwater rotifer Brachionus calyciflorus cultured in a continuous feed back mechanism in comparison with a mixed zooplankton diet, Journal of Fisheries and Aquatic Science, 2(4), 275-284
https://doi.org/10.3923/jfas.2007.275.284
Awaïss A., Kestemont K. and Micha J.-C., 1992, Nutritional suitability of the Rotifer Brachionus calyciflorus Pallas for rearing freshwater fish larvae, Journal Applied Ichthyolology, 8: 263-270
https://doi.org/10.1111/j.1439-0426. 1992.tb00693.x
Barbe J., Schlumberger O., and Bouretz N., 2000, Evaluation de la production piscicole potentielle des étangs, Ingenieries-EAT, 22: 49-62
https://hal.archives-ouvertes.fr/hal-00464073
Bokossa H.K.J., Saïdou A., Sossoukpe E., Fiogbé D.E., and Kossou D., 2014, Decomposition and Mineralization Effect of Various Sources of Pig Manure on Water Quality and Nutrients Availability for Agro-Fish System in Benin, Agricultural Sciences, 5(12): 1194-1206
https://doi.org/10.4236/as.2014.512129
Boyd C.A., Penseng P., and Boyd C.E., 2008, New Nitrogen Fertilization Recommendations for Bluegill Ponds in the Southeastern United States, North American Journal of Aquaculture, 70(3): 308-313
https://doi.org/10.1577/A07-052.1
Canovas S., Casellas C., Picot B., Pena G., and Bontoux J., 1991, Evolution annuelle du peuplement zooplanctonique dans un lagunage à haut rendement et incidence du temps de séjour, Revue des Sciences de l'Eau/Journal of Water Science, 4(2): 269-289
https://doi.org/10.7202/705100ar
Culver B.A., Boucherle M.M., Bean D.J., and Fletcher J.W., 1985, Biomass sf freshwater crustacean zooplankton from length-weight regressions, Canadian Journal of Fisheries and Aquatic Sciences, 42(8): 1380-1390
https://doi.org/10.1139/f85-173
Dalme D.K., and Chari M.S., 2011, Performance evaluation of different animal wastes on culture of Daphnia sp., Journal of Fisheries and Aquatic Sciences, 6(1): 57-61
https://doi.org/10.3923/jfas.2011.57.61
Ekelemu J.K., and Nwabueze A.A., 2011, Comparative studies on zooplankton production using different types of organic manure, International Journal of Science and Nature, 2(1), 140-143
http://www.scienceandnature.org/IJSN-Vol2(1)M2011/IJSN-VOL2(1)-30.pdf
Fiogbe E.D., Kestemont P., Micha J.C., 2003, Performances zootechniques comparées de rotifères d'eau douce Brachionus calyciflorus et de nauplies d'Artemia chez les larves de la perche fluviatile perca fluviatilis, Tropicultura, 21(1): 31-35
http://www.tropicultura.org/text/v21n1.pdf#page=33
Guiral D., Arfi R., Bouvy M., Pagano M., and Saint-Jean L., 1994, Ecological organization and succession during natural recolonization of a tropical pond, Hydrobiologia, 294(3): 229-242
https://doi.org/10.1007/BF00021296
Kabir K.A., Baly R.L., Hasan I., Naser M.D.N., and Shahadat M.D., 2010, High density rotifer culture as live food for larval rearing in Carphatcheries, World Journal of Zoology, 5: 110-114
https://scholar.google.com.hk/citations?user=--agAFoAAAAJ&hl=zh-CN
Lazzaro, X., Lacroix, G., 1995, Impact des poissons sur les communautés aquatiques (p. 648-686), In Limnologie générale, Pourriot R. et Meybeck M. Eds., Masson, Paris, p. 648-686
Liady M.N.D., Tangou T.T., Fiogbe E.D., Cauchie H.M., and Vasel J.L., 2015, About the interest of a zooplankton compartment in pond systems: methodology to study the growth kinetic of Daphnia pulex on Scenedesmus sp. Water Science and Technology, 71(10): 1436-1443
https://doi.org/10.2166/wst.2015.107
PMid:26442483
Ludwig G.M., 1994, Tank culture of sunshine bass Morone chrysops X.M., saxatilis fry with freshwater rotifer B. calyciflorus and salmon starter meal as first food sources, Journal of the world Aquaculture Society, 25(2): 337-341
https://doi.org/10.1111/j.1749-7345.1994.tb00201.x
McCauley E., 1984, The estimation of the abundance and biomass of zooplankton in samples, A manual on methods for the assessment of secondary productivity in fresh waters, pp.228-265
Mouhri K., Marsot P., Pelletier E., Loudiki M., and Saint-louis R., 1995, Effets du chlorure de tributylétain sur la croissance et le métabolisme de la diatomée marine Phaeodactylum tricornutum (Bohlin) Oceanologica Acta, 18(3): 363-370
http://archimer.ifremer.fr/doc/00097/20869/18487.pdf
Ovie S.I., and Egborge A.B.M., 2002, The effect of different algal densities of Scenedesmus acuminatus on the population growth of Moina micrura Kurz (Crustacea: Anomopoda, Moinidae). Hydrobiologia, 477(1): 41-45.
https://doi.org/10.1023/A:1021060510355
Park H.G., Lee K.W., Cho S.H., Kim H.S., Jung M.M., and Kim H.S., 2001, High density culture of the fresh water rotifer, Brachionus calyciflorus. Hydrobiologia., pp.369-374
https://doi.org/10.1023/A:1017571327829
Pourriot R, Capblancq J., and Champ P. et Meyer J.A., 1982, Écologie du plancton des eaux continentales. Masson, Paris and New York. (Softcover.) 198
https://doi.org/10.4319/lo.1984.29.6.1349
Reynolds C.S., 1984, The ecology of freshwater phytoplancton, In: Beck E, ed. Cambrige studies in ecology, Cambrige University press
Rodier J., Legube B., 2016, Analyse de l'eau, 1600
https://www.unitheque.com/Livre/dunod/Technique_et_ingenierie/L_analyse_de_l_eau-93883.html
Saint-Jean L., Bonou C.A., and Pagano M., 1991, Développement et croissance en poids de Moina (cf) micrura et de Mesocyclops ogunnus dans un milieu saumâtre tropical: les étangs de pisciculture de Layo (Côte d'Ivoire), Revue d'Hydrobiologie Tropicale, 24(4): 287-303
http://www.documentation.ird.fr/hor/fdi:35861
Saint-Jean L., and Pagano M., 1987, Tailles et poids individuels des principaux taxons du zooplancton lagunaire ivoirien : lagune Ebrié; étangs de pisciculture saumâtres de Layo, Rev. Hydrobiol. Trop, 20(1): 13-20
http://agris.fao.org/agris-search/search.do?recordID=AV2012068230
Schlumberger O., and Bouretz N., 2002, Réseaux trophiques et production piscicole en étangs fertilisés (Dordogne, France) Revue des sciences de l'eau. Journal of Water Science, 15(1): 177-192
https://doi.org/10.7202/705445ar
Sendacz S., Caleffi S., and Santos-soares J., 2006, Zooplankton biomass of reservoirs in different trophic conditions in the state of são paulo, Brazil, Brazilian Journal of Biology, 66(1B): 337-350
https://doi.org/10.1590/S1519-69842006000200016
PMid:16710526
Sipaúba-Tavares L.H., and Pereira A.M.L., 2008, Large scale laboratory cultures of Ankistrodesmus gracilis (Reisch) Korsikov (Chlorophyta) and Diaphanosoma biergei Korinek, 1981 (Cladocera). Brazilian Journal of Biology, 68(4): 875-883
https://doi.org/10.1590/S1519-69842008000400025
PMid:19197508
Sprules W.G., 1980, Nommetric multidimensional scaling analyses of temporel variation in the structure of limnetic zooplankton communities, Hydrobiologia, 69 (1): 139-146
https://doi.org/10.1007/BF00016543
UNESCO, 1966, Determination of phytosynthetic pigment in seawater, Repport SCOR UNESCO, Pers 17. Monographs on oceanographic methodology
http://unesdoc.unesco.org/images/0007/000716/071612eo.pdf
Waldbauer G.P., 1968, The consumption and utilization of food by insects. Advances in insect physiology, 5: 229-288
. PDF(858KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Richard Adande
. Mouhamadou Nourou Dine Liady
. Edmond Sossoukpe
. Emile Didier Fiogbe
Related articles
. Rabbit manure
. Optimal dose
. Zooplankton
. Plurispecific production
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)
.png)
.png)


